
Readers growing up in the late 20th Century may remember the term “Hole In The Ozone Layer.” It refers to a process called Ozone Layer Depletion, which poses a significant threat to life on the planet.
The ozone layer is defined as a thin layer of concentrated ozone gas in the stratosphere, enveloping the Earth at altitudes of 15-35 kilometers or 9-22 miles. It performs the crucial role of absorbing approximately 98% of the Sun’s harmful ultraviolet radiation before reaching the planet’s surface.
For anyone who was too young or not even born when this event occurred, here’s a very short recap of what happened:
Early in the 1970s and 1980s, the scientific community became progressively more worried about the potential harmful effects of ozone-depleting substances (ODS) on the ozone layer. It created a “hole” in the ozone layer above Antarctica that was growing at an alarming rate.
These concerns were formally addressed by the Vienna Convention for the Protection of the Ozone Layer in 1985. In turn, this convention resulted in the signing of a treaty called the Montreal Protocol on Substances that Deplete the Ozone Layer in 1987.

Representatives at the 20th Anniversary of the Montreal Protocol in Canada
It was amendments to the Montreal Protocol that resulted in the decision to formally stop the production of CFCs in all developed countries by 1996.
Subsequent actions led to the hole in the ozone layer starting to shrink, and reports from NASA in 2019 indicated that it is now the smallest it has ever been.
It is unclear when the damage will be completely repaired, but this is one example of a potentially catastrophic event that was recognized, stopped in time, and even reversed. And all of this is due to global recognition and cooperation based on scientific evidence.
You may wonder why this is still such a relevant subject and also continues to receive much attention from meteorological and general scientific communities. There are good reasons for this. The most important being the effects of ozone layer depletion on the planet.
Although this event has been caught in time and reversed, it still needs to be monitored to prevent it from getting out of control again. This experience can also serve as a blueprint of how human activities can lead to unintended environmental damage and how they can be addressed.
And this is where this article comes in. The focus of the post will be on the effects of ozone depletion, which will explain why it was addressed with such urgency on a global scale.
To do this, we first need to define what the ozone layer is, as well as its importance to the global environment. The causes of ozone depletion and the hole in the ozone layer will also be examined before we can finally address the potential effects of ozone depletion.
Ozone Layer Definition
Much discussion and debate continued to rage over the ozone layer during the past four decades, with some observers not having a clear idea of what exactly it is. As a result, it is crucial to provide a clear definition first of what precisely the ozone layer is:
What Is The Ozone Layer?

The ozone layer is a thin blanket of concentrated ozone gas surrounding the Earth at an altitude of 15 – 35 kilometers or 9 – 22 miles in the stratosphere. It performs the crucial role of absorbing approximately 98% of the sun’s dangerous ultraviolet radiation reaching the planet’s atmosphere.
Ozone gets created in the stratosphere. As high levels of UV radiation hit an oxygen molecule (O2), it creates a freed oxygen atom. The oxygen atom then combines with an oxygen molecule (O2) to form ozone (O3.)
Ozone also forms primarily over the tropics. High altitude winds then carry the ozone-rich air towards the polar regions.
The ozone layer does not just vary in height but also in thickness. It varies throughout the year, but in general, it is thinner over the equator and thicker over the polar regions.
Importance Of The Ozone Layer
The ozone layer is an extremely thin layer located in the stratosphere. At its highest concentration levels, it is also still only ten parts per million of ozone. Despite its low density, the ozone layer plays an indispensable role in protecting all life on the planet.
It absorbs the vast majority (roughly 98 percent) of all ultraviolet radiation from the sun. (The dangers of this type of radiation will be addressed shortly in the “Effects of Ozone Layer Depletion” section.)
Ozone (O3) consists of three oxygen atoms and is a relatively unstable molecule. And it is this instability that helps to protect us.
It may sound confusing, but it is both the destruction and reformation of ozone in the stratosphere that protects the Earth from UV light. It can be explained as follows.

An abundance of oxygen molecules is present in the stratosphere. When ultraviolet light from the sun hits one of these molecules, it causes it to break up into two separate oxygen atoms.
A single oxygen atom (O1) is very unstable and quickly finds an oxygen molecule (O2) to bind with, which results in the formation of an ozone molecule (O3). And it is this molecule that protects the Earth from UV radiation.
In summary: Oxygen Atom (O1) + Oxygen Molecule (O2) = Ozone (O3)
Importantly, when UV light hits an ozone molecule, it absorbs the radiation and converts it into heat instead of allowing it to pass through.
During this process, though, an oxygen atom gets stripped away again, leaving an oxygen molecule (O2) and a single oxygen atom (O1).
In summary: Ozone (O3) & UV Exposure → Oxygen Atom (O1) + Oxygen Molecule (O2)
The simultaneous creation and destruction of ozone is a natural process, but creates a fine balance where the amount of ozone produced is equal to the amount of ozone destroyed.
This process is also known as the Leaky Bucket Theory. If you compare the ozone layer to a bucket with a hole in it, the bucket will remain full as long as the amount of ozone leaking out (being destroyed) remains the same as the amount of ozone added (newly created.)
It is precisely for this reason that ozone-depleting substances, which caused more ozone to be destroyed than can be newly created, resulted in a huge imbalance and the resulting depletion of the ozone layer.
In turn, increasing amounts of dangerous ultraviolet radiation were able to reach the planet’s surface. Fortunately, the Montreal Protocol addressed the alarming rate of depletion, and the ozone layer is recovering.
But this is all evidence of just how delicate this balance is and how vital it is to be maintained to allow the ozone layer to continue protecting against UV radiation.
Causes Of Ozone Layer Depletion
There are a number of substances in the atmosphere that cause ozone breakdown or depletion. They are known as ozone-depleting substances (ODS), which can occur naturally or have an artificial origin.
Hydroxyl (OH·) and nitric oxide radicals (NO·) occur naturally in the atmosphere and are highly reactive, meaning they are short-lived but can easily react with another substance. In the stratosphere, this results in the interaction and breakdown of ozone.
The biggest cause of ozone depletion, though, is the man-made chemicals that were released into the atmosphere during the late 20th Century. Of these chemicals, chlorine and bromine are by far the biggest threats.
These substances are created by chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and other gases that were used as coolants in commercial appliances like refrigerators and air-conditioners.
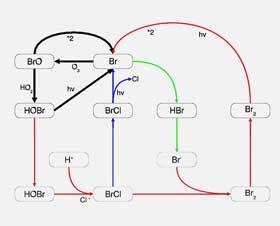
Diagram illustrating how ozone-depleting substances are destroying ozone
Once chlorofluorocarbons and hydrochlorofluorocarbons reach the stratosphere, they are exposed to ultraviolet radiation, which breaks them down into chlorine or bromine. It is in this pure form of these substances that they are able to destroy ozone on a large scale.
These chemicals are able to break down ozone by stripping away an ozone atom. Chlorine, for example, destroys ozone by reacting with and breaking the ozone molecule apart to form chlorine monoxide and oxygen.
The simple equation looks as follows: Cl + O3 = ClO + O2
It is both the capacity and lifespan of chlorine and bromine that make them so destructive. For example, one chlorine atom is able to destroy 100 000 ozone molecules. Bromine is up to 40 times more damaging than chlorine, but there is much less of it in the atmosphere.
Chlorine can also remain in the stratosphere for up to 100 years, and bromine for up to approximately 65 years. These long lifespans allow these ozone-depleting substances to remain and continue to disrupt ozone levels for a sustained period.
Effects Of Ozone Layer Depletion
The delicate balance between the creation and destruction of ozone is crucial. The depletion of ozone in the stratosphere during the late 20th Century as a result of ozone-depleting substances resulted in severe disruption of this balance.
The large-scale destruction of ozone by ozone-depleting substances in the stratosphere during the last Century resulted in more of the gas being destroyed than new ones created.
This imbalance led to an alarming decline in the amount of ozone in the stratosphere, with weaknesses known as “holes in the ozone layer” appearing over regions like Antarctica.
The amount of ultraviolet radiation that will be allowed through if the ozone layer is compromised will have devastating and deadly consequences to all life on the planet.
Ultraviolet light can be divided into three types of radiation:
- UV-A Radiation
- UV-B Radiation
- UV-C Radiation
Of these three, UV-C radiation is by far the most dangerous. It can cause severe skin burns in a short time, lead to skin cancer, and also cause permanent eye damage, which includes conditions like cataracts. Fortunately, the ozone layer blocks 100 percent of all UV-C light.
UV-B radiation is less dangerous but can still cause skin burns and is associated with certain types of skin cancers, such as carcinoma. Again, the ozone layer is able to block approximately 90 percent of all UV-B radiation.
UV-A radiation is the only type of UV light that penetrates the surface without interruption from the ozone layer. It was considered a harmless form of radiation, but recent studies reveal long-term exposure could lead to premature skin aging and cancers like Melanoma.
The point of this breakdown of ultraviolet radiation and the potential impact of its different components on human beings is to highlight just how devastating the effect of ozone depletion will be.

Just to put this in context, an article published in National Geographic in April 2019 stated that without the Montreal Protocol, “the U.S. would have seen an additional 280 million cases of skin cancer, 1.5 million skin cancer deaths, and 45 million cataracts—and the world would be at least 25 percent hotter.”
And the impact on the rest of our environment wasn’t even focused on in this section. For example, ultraviolet radiation (especially UV-B light) can also damage plants on a cellular level, alter their DNA, and even lead to plant death.
It is also important to note that animal life is also impacted in very much the same way as human life by the effects of ultraviolet radiation. Even freshwater and marine life do not escape the effects of UV light.
In summary, it is safe to state that all life on earth will be adversely affected by ozone depletion and the resulting increased ultraviolet radiation.
Conclusion
Throughout this article, especially during the last few sections, the importance of the ozone layer was highlighted, as well as how crucial it is for the delicate balance that keeps the ozone in place and protects against the vast majority of UV radiation, to be maintained.
It is important to note that, although the majority of damage done to the ozone layer by ozone-depleting substances has been stopped and even reversed, the ozone layer remains fragile and is only estimated to be fully restored by approximately 2050.
The ozone layer also remains under constant attack from existing and new threats, with new forms of chemicals released into the atmosphere, as well as global warming and climate change evolving, which may all have long-term effects on ozone that have yet to be determined.
The focus of this article was to explain what the ozone layer is and just how crucial it is for the protection of all life on the planet. It also highlighted the different causes of ozone depletion and the effect it can have on the environment and human, animal, and plant life.
Never miss out again when another interesting and helpful article is released and stay updated, while also receiving helpful tips & information by simply following this link .Until next time, keep your eye on the weather!

